CVR Medical Corp.: Company Update (CVM-TSX; CRRVF-OTCQB)
Restructuring Approved by Shareholders
 CVR Medical Corp. recently announced that its Board of Directors held a Special Meeting of the Shareholders to approve, among other things, the Restructuring of the Agreement between CVR Medical Corp. and CVR Global Inc. CVR Medical Shareholders approved the restructuring as well as all other items put forth for a vote.
CVR Medical Corp. recently announced that its Board of Directors held a Special Meeting of the Shareholders to approve, among other things, the Restructuring of the Agreement between CVR Medical Corp. and CVR Global Inc. CVR Medical Shareholders approved the restructuring as well as all other items put forth for a vote.
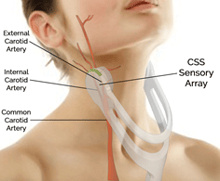 This restructured agreement calls for the companies to terminate and relinquish the previously agreed upon 50%/50% profit sharing structure in exchange for 30 million shares of CVR Medical Corp. stock and additional royalties associated with the commercialization and future sales of the Company's Carotid Stenotic Scan (CSS) as pictured here —a device which uses low frequency sound wave analysis to non-invasively detect and measure carotid arterial stenosis (narrowing of the blood vessels in the neck that carries blood from the heart to the brain) or occlusion (blockage)—which is the leading risk factor for stroke. As well, the current and future Intellectual Property portfolio pertaining to the CSS will now reside within CVR Medical. The agreement is subject to and will require TSX.V approval, which CVR Medical expects to be forthcoming.
This restructured agreement calls for the companies to terminate and relinquish the previously agreed upon 50%/50% profit sharing structure in exchange for 30 million shares of CVR Medical Corp. stock and additional royalties associated with the commercialization and future sales of the Company's Carotid Stenotic Scan (CSS) as pictured here —a device which uses low frequency sound wave analysis to non-invasively detect and measure carotid arterial stenosis (narrowing of the blood vessels in the neck that carries blood from the heart to the brain) or occlusion (blockage)—which is the leading risk factor for stroke. As well, the current and future Intellectual Property portfolio pertaining to the CSS will now reside within CVR Medical. The agreement is subject to and will require TSX.V approval, which CVR Medical expects to be forthcoming.
With the approved transaction, CVR Global Inc. is expected to move into a milestone-driven equity-based contractor and, upon achieving the milestones stated below, could become one of the Company’s largest shareholders. These milestones include the:
- Approval and signing of agreement (3 million shares)
- FDA submission (2 million shares)
- FDA clearance/approval (10 million shares)
- Achievement of $50 million in sales from CSS Device Sales (15 million shares)—contingent on a maximum 36-month time frame from initial CSS sale
CVR Global is further expected to be granted a 7% royalty on all CSS device sales, with a 3% royalty on all associated disposable sales. With this transaction, roles are now clearly defined along with the pathway to the CSS market launch.
Announces Proposed Financing
In other recent news, CVR Medical announced that it intends to complete a non-brokered private placement financing of units for gross proceeds of up to C$2 million with a price per Unit of C$0.30 or such other price determined by CVR Medical management in compliance with TSX Venture Exchange pricing regulations.
 Each Unit is to consist of one common share and one common share purchase warrant, with each Warrant exercisable to acquire one additional Share at a price of C$0.36 per Warrant Share for a period of five years following the closing date of the Financing. The Warrants will be subject to an acceleration right if on any ten consecutive trading days, beginning on the date that is four months and one day following the Closing Date, the daily closing price of the Shares on the Exchange is at or greater than C$0.50. If the Company exercises its Warrant Acceleration Right, the new expiry date of the Warrants will be the 30th day following the date on which such notice is given by the Company. The Company expects to pay finder’s fees of up to 6% in cash and 6% in finder’s warrants in connection with the Financing.
Each Unit is to consist of one common share and one common share purchase warrant, with each Warrant exercisable to acquire one additional Share at a price of C$0.36 per Warrant Share for a period of five years following the closing date of the Financing. The Warrants will be subject to an acceleration right if on any ten consecutive trading days, beginning on the date that is four months and one day following the Closing Date, the daily closing price of the Shares on the Exchange is at or greater than C$0.50. If the Company exercises its Warrant Acceleration Right, the new expiry date of the Warrants will be the 30th day following the date on which such notice is given by the Company. The Company expects to pay finder’s fees of up to 6% in cash and 6% in finder’s warrants in connection with the Financing.
Proceeds are expected to be used for ongoing working capital requirements related to the development and commercialization of the Company’s CSS Device. Completion of the Financing is subject to Exchange acceptance and all securities issued pursuant to the Financing will be subject to a hold period of four months as required under applicable securities legislation.
Positive Interim Results Reported From Ongoing Pivotal Trial With CSS Device
The Company further announced advisory staff member Dr. Phillip J. Bendick, PhD, has released an internal report summarizing data from the pivotal clinical trials for the CSS devices in use at Thomas Jefferson University Hospital. In alignment with the previous report released by Dr. Bendick in September 2017, this preliminary report views the initial batch of data points collected within the trial as successful for the substantiation of the device’s value and efficacy.
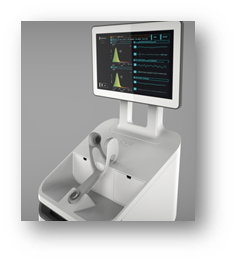 With the trial ongoing (thus only a partial snapshot of the data), the improvements made to the CSS prior to launch of the pivotal clinical trials has generated enhanced functionality within the clinical setting. The CSS Device is being evaluated in a multi-center pivotal clinical trial to evaluate the system’s specificity and sensitivity for detecting carotid artery disease, compared to the current gold standard – duplex Doppler ultrasound. According to Dr. Bendick, “The early results of the first 144 patients enrolled in the CSS pivotal trial have shown excellent overall accuracy […] these initial results demonstrate the efficacy of the CSS as a tool to detect and guide the appropriate management of clinically important carotid artery disease in order to reduce stroke risk.”
With the trial ongoing (thus only a partial snapshot of the data), the improvements made to the CSS prior to launch of the pivotal clinical trials has generated enhanced functionality within the clinical setting. The CSS Device is being evaluated in a multi-center pivotal clinical trial to evaluate the system’s specificity and sensitivity for detecting carotid artery disease, compared to the current gold standard – duplex Doppler ultrasound. According to Dr. Bendick, “The early results of the first 144 patients enrolled in the CSS pivotal trial have shown excellent overall accuracy […] these initial results demonstrate the efficacy of the CSS as a tool to detect and guide the appropriate management of clinically important carotid artery disease in order to reduce stroke risk.”
******************************************************
Learn move about CVR Medical by downloading our Executive Informational Overview (EIO), a 60-page report detailing the Company's business, product development, strategic relationships, market opportunities, competition, financials, risks, and more.
Snapshot
CVR Medical Corp. is a medical technology company developing and commercializing a revolutionary device to assess carotid arterial health. The technology is intended to quickly and cost-effectively identify patients with arterial narrowing, which puts them at risk for ischemic stroke, in order to enable early intervention. Performed in a primary care physician’s office, the Company’s Carotid Stenotic Scan (CSS) uses low frequency sound wave analysis to non-invasively detect and measure carotid arterial stenosis (narrowing of the blood vessels in the neck that carries blood from the heart to the brain) or occlusion (blockage)—which is the leading risk factor for stroke. The CSS, which can be performed within two minutes by a medical technician in the office, costs less than invasive diagnostics, such as duplex Doppler ultrasound (DUS), magnetic resonance angiography (MRA), computed tomography angiography (CTA), or cerebral angiogram, and provides results at the point of care. Stroke is a very serious medical condition that requires immediate emergency care as it can cause lasting brain damage, long-term disability, or death. Primary care physicians currently lack a cost-effective tool to initially assess their patients’ arterial health. The CSS device is undergoing final phase trials at several world-renowned medical institutions and is positioned for imminent U.S. FDA submission, expected to be followed by market clearance and launch—targeted for 1H 2019.
Key Points of CVR Medical Corp.'s Business
- Stroke is the fifth leading cause of death in the U.S., resulting in one out of 20 adult deaths, at a cost of roughly $34 billion per year. Many of these deaths could be preventable. While cardiac disease is the primary cause of death, stroke is the leading cause of long-term disability (50% of patients are permanently impaired).
- The Company announced in January 2018 the start of clinical trials at the Henry Ford Hospital in Detroit. Additionally, Thomas Jefferson University Hospital in Philadelphia is expected to begin pivotal trials following promising preliminary results of its ongoing ENTICES Study, evaluating CSS against current ultrasound technologies. Furthermore, in February 2018, the Company announced Internal Review Board (IRB) approval by the Cleveland Clinic, sanctioning CVR Medical to conduct clinical trials using the CSS device.
- CVR Medical has a contract with Canon U.S.A. to build and manufacture its CSS device, where Canon will manufacture, assemble, handle all logistics (including tech support), and ‘white glove’ deliver the CSS to the doctor’s office. Canon brings significant value to the Company as it has a dedicated space with the ability to scale production as CVR Medical transitions into a full scale medical device sales and marketing company.
- CVR Medical’s intellectual property (IP) portfolio includes one issued patent, 13 pending, and more than 20 under process, with significant IP surrounding its CSS, including 4K encrypted proprietary software.
- The Company’s executive team consists of veterans from the medical device industry with business acumen from key areas in healthcare.
*******************************************
Visit our Corporate Profile and Key Points pages
for the latest research on CVR Medical Corp.
*******************************************

